Anna Cociurovscaia1, Grzegorz Bujacz1, Agnieszka Pietrzyk-Brzezińska1
1Institute of Molecular and Industrial Biotechnology, Lodz University of Technology, 90-537 Lodz, Poland
anna.cociurovscaia@dokt.p.lodz.pl
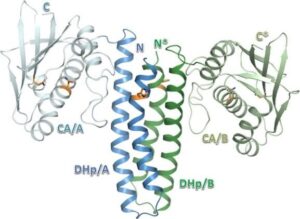
The Escherichia coli CusS histidine kinase belongs to the bacterial two-component regulatory system CusS-CusR, engaged in sensing copper ions excess and activating the transcription of chemiosmotic efflux pump CusCFBA.1 The CusS like classical histidine kinase is a transmembrane multidomain protein.2 It binds copper by periplasmic sensor domain and propagates this signal toward the cytoplasmic catalytic core, through the coordinated conformational change of its subsequent domains. Then, the kinase core binds ATP, autophosphorylates its conserved histidine residue and transmits the γ-phosphoryl group to its cognate response regulator CusR. As a consequence phosphorylatably induced response regulator binds to the target operon, responsible for the synthesis of copper-efflux pomp, regulating its transcription.3 A small amount of copper ions is indispensable for catalytic properties of enzymes involved in aerobic cell metabolism. Nonetheless, free copper ions excess in the cytoplasm generates damaging reactive hydroxyl radicals.4 For that reason, understanding bacterial copper sensing mechanism can contribute to reducing bacterial resistance and developing of bactericidal copper-based materials. Using the X-ray crystallography, the crystal structure of the CusS kinase core was solved at the resolution of 1.4 Å. The cytoplasmic catalytic domains ensemble in a homodimer structure. It allowed studying intramolecular and intermolecular interactions crucial for the mechanism of CusS autophosphorylation. Based on obtained structural data, conserved catalytic and structural motifs were identified and described. All conserved motifs classify CusS into the Type I family of histidine kinases, the most common group of these enzymes constituting 72% of all histidine kinases.
References:
[1] G.P. Munson, D.L. Lam, F.W. Outten, T. V. O’Halloran, J. Bacteriol., 182, (2000), 5864–5871.
[2] T. Affandi, A. V. Issaian, M.M. McEvoy, Biochemistry., 55, (2016), 5296–5306.
[3] G. Grass, C. Rensing, J. Bacteriol., 183, (2001), 2145–2147. 4. A. Giachino, K.J. Waldron, Mol. Microbiol., 114, (2020), 377–390.
The abstract was produced as part of the Doctoral Candidate’s participation in a project of the National Agency for Academic Exchange within the framework of the “STER” Programme. – Internationalisation of Doctoral Schools” as part of the project “Curriculum for advanced doctoral education & training – CADET Academy of Lodz University of Technology”.
Keywords: histidine kinase; two-component signal transduction system; autophosphorylation; crystal structure; Escherichia coli
