Uršula Vide1, Martin Paul Heimböck1, Elfriede Zenzmaier1,
Andreas Winkler1
1Institute of Biochemistry, Graz University of Technology, Graz, Austria
into a coiled-coil.
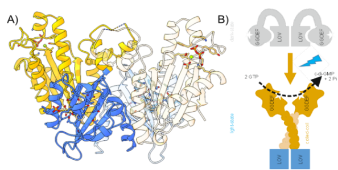
Perception of light is an important aspect of survival in a competitive environment, thus a plethora of photoreceptors have evolved in different organisms. Photoreceptors sensing blue light were early on identified as key players in plant phototropism, and belong to the family of flavin-dependent light-oxygen-voltage (LOV) domains.1 LOV domains have a high potential in applied biosciences, since they can be used as optogenetic tools.2 In this study, we characterized a LOV-activated diguanylate cyclase (LadC) that offers precise temporal and spatial control of enzymatic activity with an exceptionally high dynamic range over four orders of magnitude making it a switch-like system. We employed an integrative structural biology approach combining X-ray crystallography, solution scattering, computational methods and hydrogen-deuterium exchange coupled to mass spectrometry to reveal how this protein switch operates on a molecular level. To solve the full-length crystal structure of dark-adapted LOV-GGDEF system we adapted the strategy of refinement due to so far unidentified crystal pathology indicated in Wilson distribution of reflection’s intensities. The structure (Fig.1A) reveled that the dark, inhibited conformation is characterized by a tight association of the sensory LOV and catalytic GGDEF domains in the dimeric assembly, which prevents the productive encounter of two GGDEF domains that would be required for catalysis. The efficient caging of the effector domains is only released upon structural rearrangements induced by blue light illumination (Fig.1B).
Our work aims to understand the mechanism behind how LadC changes its enzymatic activity in response to light, with a focus on its potential use in optogenetics and other applications that require precise temporal and spatial control of enzymatic activity or other biological outputs.
References:
[1] Losi & Gärtner (2017) Photochem Photobiol 93: p. 141-158.
[2] Pudasaini et al. (2015) Front. Mol. Biosci. 2:18.
Keywords: photoreceptors; blue light; diguanylate cyclase; optogenetics; allosteric regulation
