HT1: Crystallization
L3 (Lecture): A protein crystallization strategy for screening and optimization for structure-based drug design
Terese Bergfors
Crystallization Platform, Dept. of Cell and Molecular Biology
Uppsala University
Sweden
Structure-based drug design depends on a steady supply of protein crystals containing the small molecules of interest. Often the small molecules are poorly soluble and in shorter supply than the protein itself. The protein may not crystallize with the ligand, and sometimes it won’t crystallize without it. What would be a rational crystallization strategy to deal with these and other crystallization problems?
My answers are from the perspective of an academic laboratory that works on proteins in the non-mevalonate pathway of multiple pathogens. I will present examples of our work on proteins from this pathway in Mycobacterium tuberculosis (tuberculosis), Plasmodium spp. (malaria), and ESKAPE bacteria (implicated in hospital-acquired infections). Our initial screen contains 192 conditions and our first-choice optimization method is matrix microseeding.
Among other questions, the talk will discuss: What is the single most important parameter in screening? How important is the protein formulation itself in the screening process? Are all crystallization kits equally successful, or are some better than others? Which kind of leads are worth optimizing, and how should that be done? How similar do homologous proteins have to be for cross-seeding to work?
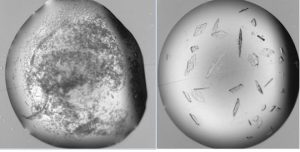
Figure.1. Cross-seeding with a homologous protein. The Plasmodium falciparum IspC protein would not crystallize, so it was cross-seeded with the P. vivax IspC crystals. The two homologues share 70% sequence identity. Left, 1 hour post-seeding. Right, 12 hours later. Crystals were 0.15 mm in the longest direction and diffracted to 1.7Å.
References
- Sooriyaarachchi, S., Bergfors, T., et al. (2016) ChemMedChem 11, 1-14.
- D’Arcy, A., Bergfors, T., Cowan-Jacob, S.W. and Marsh, M. (2014) Acta Cryst F70, 1117-1126.
